FebriDx® Bacterial / Non-Bacterial POC Test

FebriDx is a rapid, self-contained test that aids in the diagnosis of acute respiratory infection and differentiates bacterial from non-bacterial etiology after 10 minutes.
- FDA 510(k) cleared
About
FebriDx is a rapid, self-contained test that uses a simple fingerstick to aid in the diagnosis of acute respiratory infection and differentiate bacterial from non-bacterial etiology. It uses dual biomarkers—MxA, which signals a viral immune response, and CRP, which rises with inflammation and infection—to deliver trusted results after 10 minutes. No instruments, lab equipment, or readers required. FDA 510(k) cleared with 99% NPV to confidently rule out bacterial infection.1
1. Shapiro NI, Filbin MR, Hou PC, et al. Diagnostic accuracy of a bacterial and viral biomarker point-of-care test in the outpatient setting. JAMA Netw Open, 2022, 5(10): e2234588.
Specifications
| Product code | P0211 |
|---|---|
| Contents | Integrated single-use test devices (containing lancet, buffer and test strip), instructions for use, quick reference guide (QRI) |
| Tests per kit | 25 |
| Specimen type | Fingerstick blood |
| Time to result | After 10 minutes |
| Clinical performance (vs reference methods) | 99% NPV to rule out bacterial infection |
| Biomarkers | MxA (viral biomarker indicating immune response to viral infection) CRP (biomarker associated with acute inflammation and infection) |
| FDA clearance | FDA 510(k) cleared (K230917) |
| Shelf life | 24 months |
| Storage condition | 39-77ºF (4-25ºC) |
Intended use
FebriDx is a rapid, self-contained, point-of-care immunoassay intended for the qualitative detection of myxovirus resistance protein A (MxA) and C-reactive protein (CRP) from fingerstick blood samples to aid in the diagnosis of acute respiratory infection and differentiate bacterial from non-bacterial etiology.
Frequently Asked Questions
Patients aged 12 to 64 who present to urgent care or emergency care settings for evaluation of acute respiratory infection who have had symptoms for less than 7 days and within 3 days of fever onset.
Fresh capillary blood (fingerstick) must be used on the FebriDx test. Venous blood, serum and/or plasma cannot be used.
PLA0442U: This FebriDx Proprietary Laboratory Assessment (PLA) code is published on the Clinical Lab Fee Schedule, effective January 1, 2025.
Do not read results before 10 minutes or after 1 hour. Reading results before the blood has cleared the Result Window or without blood in the Blood Clearance Window may lead to erroneous test results.
FebriDx has a 99% NPV to rule out bacterial infection. Refer to the Instructions for Use (PM-127) for the complete performance data.
FebriDx utilizes proprietary dual biomarker technology: C-Reactive Protein (CRP) and Myxovirus resistance protein A (MxA).
→ CRP: 20 µg/mL
→ MxA: 40 ng/mL
FebriDx tests should be stored at room temperature (4-25°C or 39-77°F).
Unopened FebriDx tests are stable up to 24 months from the date of manufacture when stored at a temperature between 39 - 77°F (4 - 25°C).
FebriDx is packaged in a kit box containing 25 tests. There are 100 FebriDx tests (4 kit boxes) per case.
The FebriDx test is a qualitative visually read rapid immunoassay.
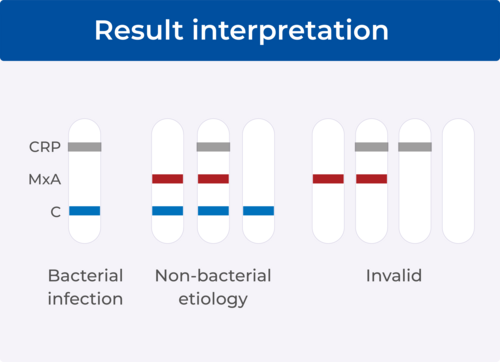
No. A blue control line must appear in the result window for the test to be valid. The absence of the blue control line indicates an invalid test, and the patient must be retested with a new FebriDx test.
No. Even if the result line is faint in color, incomplete over the width of the test strip or uneven in color, it should be interpreted as present.
Yes. Any test with a red result line (MxA) or no result line and a blue control line is interpreted as a non-bacterial etiology. A blue control line shows that the test is valid. The absence of the blue control line indicates an invalid test.
Yes. Contact PHASE Scientific at 657-233-5880 or USsales@phasesci.com to order FebriDx External Controls (P0212).
Yes. FebriDx aids in the diagnosis of bacterial acute respiratory infection and differentiation from nonbacterial etiology and has the potential to improve diagnostic certainty and support clinical and therapeutic management decisions.
1. FebriDx Bacterial / Non-Bacterial Point-of-Care Assay; Lumos Diagnostics Instructions for Use (PM-127); FDA 510(k)K230917
2. Shapiro NI, Filbin MR, Hou PC, Kurz MC, Han JH, Aufderheide TP, Ward MA, Pulia MS, Birkhahn RH, Diaz JL,Hughes TL, Harsch MR, Bell A, Suarez-Cuervo C, Sambursky R. Diagnostic Accuracy of a Bacterial and Viral Biomarker Point-of-Care Test in the Outpatient Setting. JAMA Netw Open. 2022 Oct 3;5(10):e2234588. doi:10.1001/jamanetworkopen.2022.34588. PMID: 36255727; PMCID: PMC9579916.
3. American Hospital Association: https://www.aapc.com/codes/cpt-codes/0442U
FebriDx Bacterial / Non-Bacterial POC Test Downloads
FebriDx Bacterial / Non-bacterial POC Test Sales Sheet
FebriDx Bacterial / Non-bacterial POC Test FAQs
FebriDx Bacterial / Non-bacterial POC Test Instructions for Use PM-127 (IFU)
Please see Instructions for Use (PM-127) for the detailed steps on how to perform the test and interpret results.
(lancet included in device)
Contact Sales & Customer Service

ussales@phasesci.com

Call Sales
+1 (657) 233-5880

Customer Service
+1 (657) 296-6106
