INDICAID® COVID-19 / Influenza A&B Antigen Test

FDA authorized 3-in-1 rapid POC test with exceptional accuracy, results in 15 minutes
- FDA authorized
- For in vitro diagnostic use
- For professional use
- For use with anterior nasal swab specimens
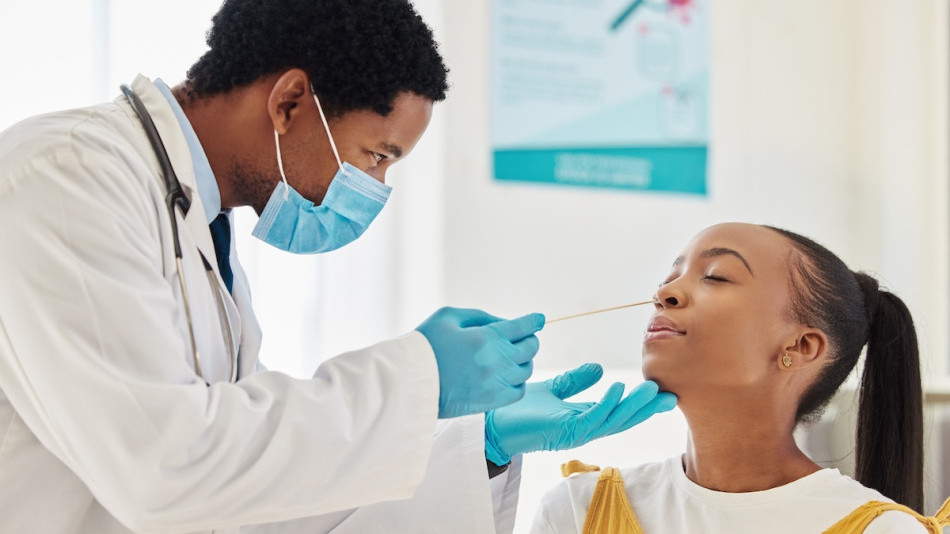
About
The COVID-19 / Influenza A&B Antigen Test is an innovative rapid test designed to qualitativley detect and differentiate influenza A and B nucleoprotein antigens and SARS-CoV-2 nucleocapsid antigen.
Features
- 3-in-1 detection: Detects COVID-19, Influenza A and Influenza B from a single nasal swab
- Fast results: Provides results in just 15 minutes
- Highly accurate: COVID-19 sensitivity: 92%, Influenza A sensitivity: 92.5%, and Influenza B sensitivity: 90.5%
- Easy sampling: Utilizes a shallow nasal swab and suitable for individuals aged 14 years or older testing themselves, or adults testing individuals aged 2 years or older
Specifications
| Contents | Individually packaged test cassettes, buffer tubes and swabs, tube holders, quick reference guide, instructions for use |
|---|---|
| Tests per kit | 25 |
| Specimen type | Anterior nasal swab |
| Time to result | 15 minutes |
| Clinical performance (vs RT-PCR) | Sensitivity (PPA) COVID-19: 99.0% | FLU A: 99.9%. | FLU B: 99.9% |
| FDA authorization | FDA De Novo granted |
| Storage condition | 36º-86ºF (2º-30ºC); do not freeze; avoid direct sunlight |
| Shelf life | 18 Months |
Intended use
For in vitro diagnostic use. For professional use.
This test is intended for the qualitative detection and differentiation of influenza A, and influenza B nucleoprotein antigens and SARS-CoV-2 nucleocapsid antigen directly in anterior nasal swab samples from individuals with signs and symptoms of respiratory tract infection.
All negative results are presumptive and should be confirmed with an FDA-cleared molecular assay when determined to be appropriate by a healthcare provider. Negative results do not rule out infection with influenza and SARS-CoV-2 or other pathogens. Individuals who test negative and experience continued or worsening respiratory symptoms, such as fever, cough and/or shortness of breath, should therefore seek follow-up care from their healthcare provider.
Positive results do not rule out co-infection with other respiratory pathogens and therefore do not substitute for a visit to a healthcare provider or appropriate follow-up.

INDICAID COVID-19 / Influenza A&B Antigen Test DOWNLOADS
Contact Sales & Customer Service

ussales@phasesci.com

Call Sales
+1 (657) 233-5880

Customer Service
+1 (657) 296-6106
