INDICAID OTC COVID-19 Rapid Antigen Test

INDICAID OTC Delivers Options for Families, Individuals, and more.
- FDA EUA: 220152
- HSA/FSA ELIGIBLE
- NDC NUMBERS: 60008-0407-80 (2-Pack)
- For In Vitro Diagnostic Use Only
- Personal Non-prescription Use
About

INDICAID is a fast, reliable, and affordable COVID-19 Rapid Antigen At-Home Test for use by your patients, customers, and employees wanting to know if they may have contracted COVID-19, by living their daily lives.
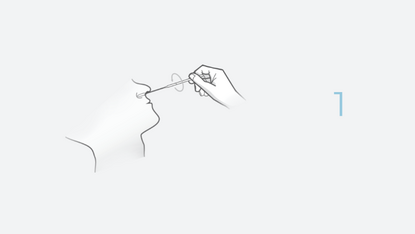
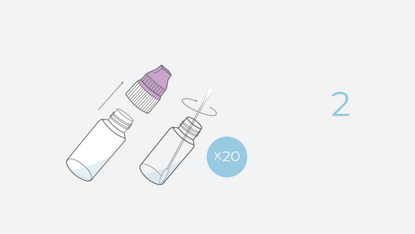
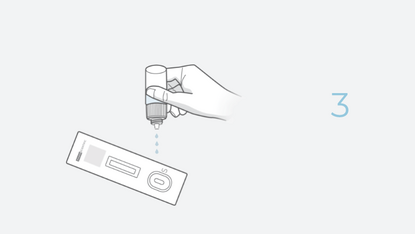
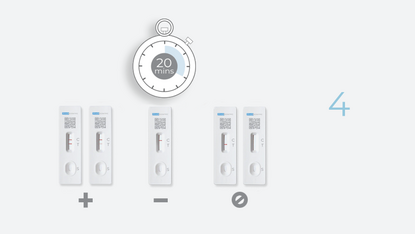
4 Steps. 20 Minutes. 0 Discomfort.

Hospitals, Urgent Care & Physician Clinics
Send patients home with a sufficient self-testing supply for continued at-home monitoring.

Workplace & Employee Wellness
Convenient for your employees to test at-home quickly and easily, with rapid and accurate results.

Retail & Pharmacy
Help customers get fast, accurate results with at-home, DIY testing.
INDICAID OMICRON DETECTION STATEMENT
“PHASE Scientific continuously monitors the performance
of our test against emerging COVID-19 variants. To date, in-house analytical
studies have not identified any variants of concern that have an impact to the
performance of INDICAID, including Omicron sub-variants BA.4, BA.5, BQ.1, BQ1.1, XBB.1.5, and XBB.1.16.
View our Omicron Statement Update

It Works & It's Simple.
I bought the test to go to my nephew's birthday. It was super easy to use, and totally straight forward. Got my results done fast and was off to the party. Would totally recommend and will be using it again.
Amazon Customer
Amazon Verified Review
Specifications
| Product Code | EUA220152 |
|---|---|
| Items Per Box | 2 |
| HSA/FSA | ELIGIBLE |
| Speciman Type | Direct Anterior Nasal Swab |
| Storage Condition | 36º-86ºF (2º-30ºC) Do not freeze. Avoid direct sunlight. |
| Shelf Life | 15 Months (Includes 15 Month FDA Extension. Learn More. |
| Accuracy | Sensitivity 81.7%; Specificity 99.4%; Overall accuracy 93.4% |
Intended Use
The INDICAID COVID-19 Rapid Antigen At-Home Test is intended for non-prescription use with self-collected anterior nasal (nares) swab samples from individuals aged 14 or older, or an adult lay user testing another aged 2 years or older. This test is authorized for individuals with symptoms of COVID-19 within the first 6 days of symptom onset when tested at least twice over three days with at least 48 hours between tests, and for individuals without symptoms or other epidemiological reasons to suspect COVID-19, when tested at least three times over five days with at least 48 hours between tests. All negative results are presumptive. The INDICAID COVID-19 Rapid Antigen At-Home Test is only for in vitro diagnostic use under the Food and Drug Administration’s Emergency Use Authorization. This product has not been FDA cleared or approved.
Principle
Antigens are present in the SARS-CoV-2 virus, and can bound with specific antibodies.
When a virus enters a human body and begins to multiply, the body begins to react to the viral antigens, possibly resulting in symptoms.
The INDICAID COVID-19 Rapid Antigen At-Home Test Rapid detects antigens from SARS-CoV-2 virus and can be used for COVID-19 screening during active infection
Frequently Asked Questions
The shelf-life of the INDICAID test is 15 Months from the date of manufacture. The expiration date is provided on the label of the outer box. This includes the 3-Month (90 day) FDA extension. Learn more.
Data from our US multi-center clinical study demonstrates similar performance for detection of the COVID-19 Omicron variant compared to the original COVID-19 strain.
The swab test method is utilized by the biomedical community for the collection of upper-respiratory specimens for molecular and rapid antigen testing. The CDC supports and promotes this testing method in light of the current COVID-19 pandemic climate for its practicality, simplicity, and efficiency of gathering much-needed data in bulk.
There are different kinds of tests for COVID-19. Molecular tests (also known as PCR tests) detect genetic material from the virus. Antigen tests detect proteins from the virus. Antigen tests are very specific for the virus but are not as sensitive as molecular tests. This means that a positive result is highly accurate, but a negative result does not rule out infection. Due to the lower sensitivity of antigen tests, there is a higher chance this test will give you a false negative result when you have COVID-19 than a molecular test would.
If your antigen test result is negative, serial testing should be performed at least twice over three days (with 48 hours between tests) for symptomatic individuals and three times over five days (with at least 48 hours between tests) for asymptomatic individuals. You may need to purchase additional tests to perform this serial (repeat) testing. All negative results are presumptive.
Discuss with your healthcare provider whether a molecular test would help with your care, and when you should discontinue home isolation if necessary. If a molecular test is not available, the CDC recommends that you should stay home.
Refer to the CDC website for most updated guidelines: https://www.cdc.gov/coronavirus/2019-ncov/your-health/isolation.html
Antigen tests are known to be less sensitive than molecular tests that detect viral nucleic acids. Negative results should be treated as presumptive and may be confirmed with a molecular assay, if necessary, for patient management. Results should be considered in the context of a patient’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19.
• This test does not differentiate between SARS-CoV and SARS-CoV-2 viruses, so you may receive a positive result but have a different coronavirus that does not cause COVID-19.
• It is possible to test a person too early or too late during COVID-19 to make an accurate diagnosis. The amount of antigen in a sample may decrease as the duration of illness increases. Negative results should be treated as presumptive. Results from patients with symptom onset beyond 5 days may need to be confirmed with a molecular assay, if necessary, for patient management.
• Improper handling of the test kit not according to guidelines may yield imprecise test results.
• When an incorrect result is suspected, it is important that you engage with your healthcare provider to help you understand the situation and evaluate your next steps.
A negative test result means that proteins from the virus that causes COVID-19 were not found in your sample, all negative results are presumptive. However, it is possible for this test to give a negative result that is incorrect (false-negative) in some people with COVID-19. Your healthcare provider will consider the test result together with all other aspects of your medical history (such as symptoms, possible exposures, and geographical location of places you have recently traveled) in deciding how to care for you.
Serial testing should be performed in individuals with negative results at least twice over three days (with 48 hours between tests) for symptomatic individuals and three times over five days (with at least 48 hours between tests) for asymptomatic individuals. You may need to purchase additional tests to perform this serial (repeat) testing.
A positive test result means that proteins from the virus that causes COVID-19 were found in your sample. This means that it is likely that you have COVID-19, even if you do not have any symptoms. There is also a very small chance that this test can give a positive result that is incorrect (a false-positive), particularly when used in a population without many cases of COVID-19. Your healthcare provider will discuss the next steps with you and how to best care for you based on your medical history and symptoms.
The INDICAID COVID-19 Rapid Antigen Test is for diagnosis only and is safe to use, with no long-term impact on human health.
Potential risks include:
Possible discomfort or other complications that can happen during sample collection.Possible incorrect test result (see below for more information.
Potential benefits include:
The results, along with other information, can help your healthcare provider make informed recommendations about your care.The results of this test may help limit the spread of COVID-19 to your family and those you come in contact with.
For detailed information see the “Warnings, Precautions, and Safety Information” section of the Instructions For Use (IFU).
This test can be administered with no equipment or training needed. The test has high sensitivity and can detect lower viral load samples against competitive products. This test has been granted Emergency Use Authorization (EUA) by the United States Food and Drug Administration.
The INDICAID COVID-19 Rapid Antigen Test is a lateral flow immunoassay designed for the qualitative detection of SARS-CoV-2 antigens. Antigen tests are designed to detect proteins from the virus that causes COVID-19 through swab specimens taken from the patient’s nose.
INDICAID OTC DOWNLOADS
NDC NUMBERS:
60008-0407-80 (2-Pack)
INDICAID OTC Rapid Antigen Test Instruction Guide

Reading Test Results
You can find the results diagram in the Instructions For Use (IFU) enclosed in your INDICAID® OTC box.
Report Your Test Result(s) at MakeMyTestCount.org – this voluntary and anonymous reporting helps public health teams understand COVID-19 spread in your area and across the country and informs public health decisions.
Do You Have Questions? Check our FAQ’s, or connect with a customer support representative using information at the bottom of this page or on our contact page.
Test Result Interpretation
| CT Indicator | Result | |
|---|---|---|
 | A line appears in regions (C) and (T) | Positive |
 | A line appears in region (C) | Negative |
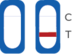 | No line appears in region (C) | Invalid |
Positive
If the control (C) line and the test (T) line are visible, the test is positive. Any faint visible red colored test (T) line with the control (C) line should be read as positive. No serial testing is required if you get a positive result at any point.
Negative
If the control line (C) is visible, but the test (T) line is NOT visible, the test is negative.
To increase the chance the negative result for COVID-19 is accurate, you should:
- Test again in 48 hours if the individual has symptoms on the first day of testing.
- Test 2 more times at least 48 hours apart if the individual does not have symptoms on the first day of testing.
Invalid
No red-colored line next to the "C" means the test is invalid. Re-test with a new swab and a new test device.

PURCHASING INDICAID® OTC COVID-19 RAPID ANTIGEN AT-HOME TESTS JUST GOT EASY…BUY ONLINE!
Today, you can order INDICAID OTC COVID-19 RAPID ANTIGEN AT-HOME Tests in 2 Packs online, in our fast and easy online store. Or, ask for INDICAID OTC at your local retail store. We are partnering with the best retailers, so you can protect yourself, and your loved ones.
CONTACT SALES & CUSTOMER SUPPORT
If you have questions about your INDICAID OTC COVID-19 Rapid Antigen At-Home Tests, first review your Instructions For Use, found in your product box, or above in our Resource section.
Need additional help? Take a look at our FAQs or Consumer Fact Sheet. These resources answer our most common questions. If you still need help, send us an email or call our support team using the information below.

Customer Service
+1 (877) 934-9355
(OPTION 1 FOR CONSUMER INQUIRIES)