INDICAID COVID-19 Rapid Antigen PoC (Point-of-Care) Test
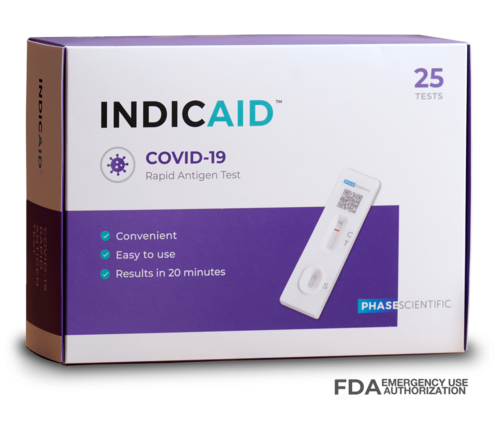
A Reliable Indicator for COVID-19 Detection for Professional Use
- FDA EUA: 210259
- For In Vitro Diagnostic Use Only
- Professional Use Only
- CLIA Number Required For Purchase
About
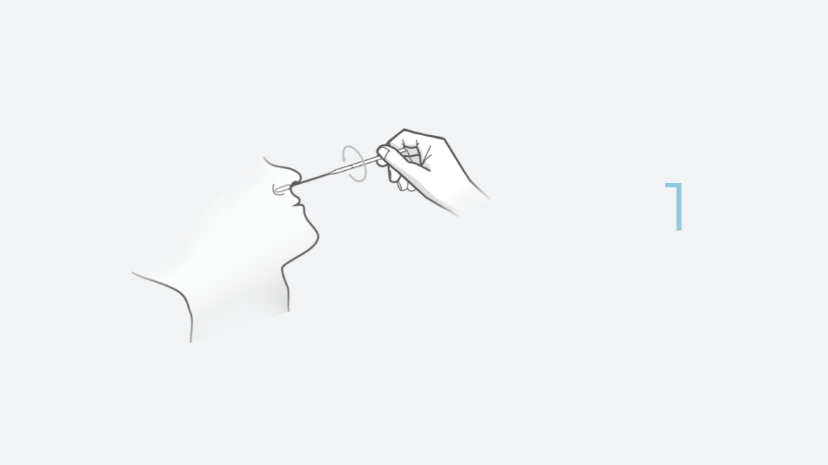
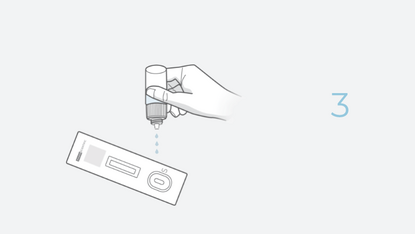
The INDICAID (Professional) COVID-19 Rapid Antigen Test (FDA EUA) is a lateral flow immunoassay designed for the qualitative detection of SARS-CoV-2 antigens in direct nasal swab samples.
Features
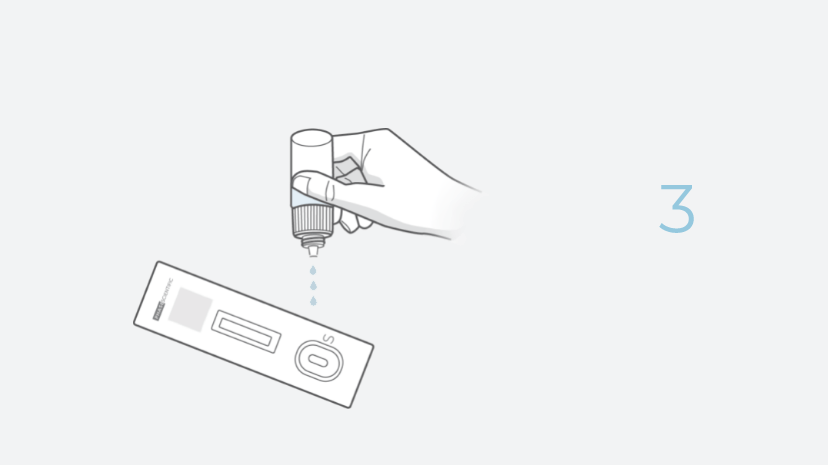
- Quick: With INDICAID, users can expect quick results in just 20 minutes.
- Easy: User-friendly testing. No additional equipment or training is required.
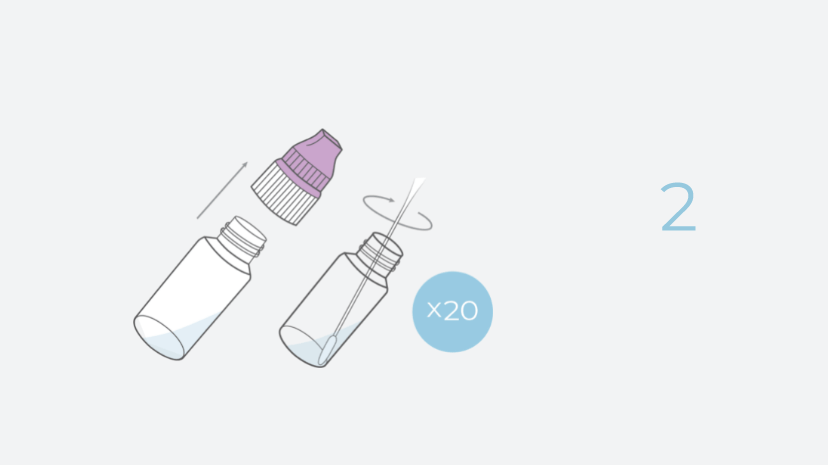
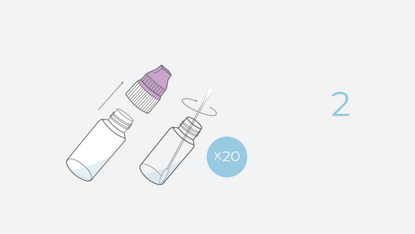
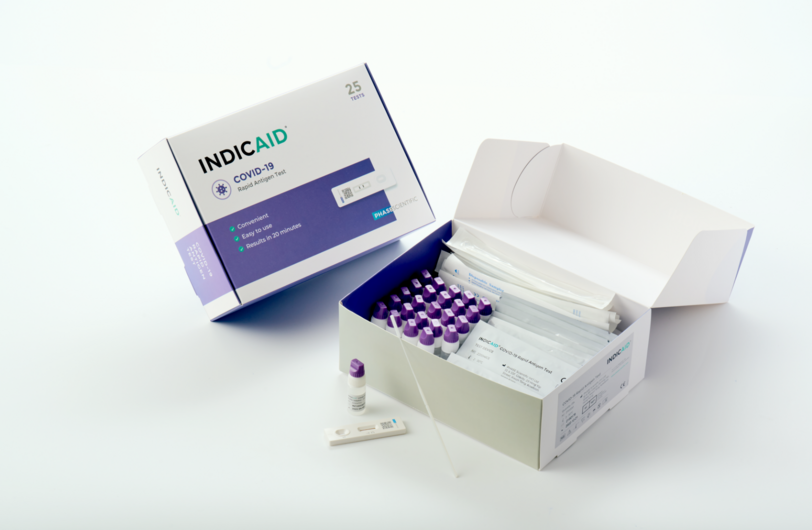
- Simple Sampling: INDICAID comes with intuitive vials to obtain self-collected shallow nasal samples.
- Bulk-Testing: Our rapid COVID-19 test allows for the aggregate collection of multiple specimens followed by batch-testing of individual samples up to two (2) hours after collection.
- Detects COVID-19 subvariants: To date, in-house analytical studies have not identified any variants of concern that have an impact to the performance of INDICAID, including Omicron sub-variants BA.4, BA.5, BQ.1, BQ.1.1, XBB.1.5, and XBB.1.16.
References
1 U.S. FDA EUA
INDICAID COVID-19 Rapid Antigen Test obtained U.S. FDA EUA in July 2021
2 Able to detect coronavirus variants
Detectable variants include Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), Delta Plus (AY.1 and AY.2), Omicron (B.1.1.529) (multiple variants), BA.2.12.1, BA.3, BA.4, BA.5, and Q.1/BQ.1.1, XBB.1.5, and XBB.1.16.
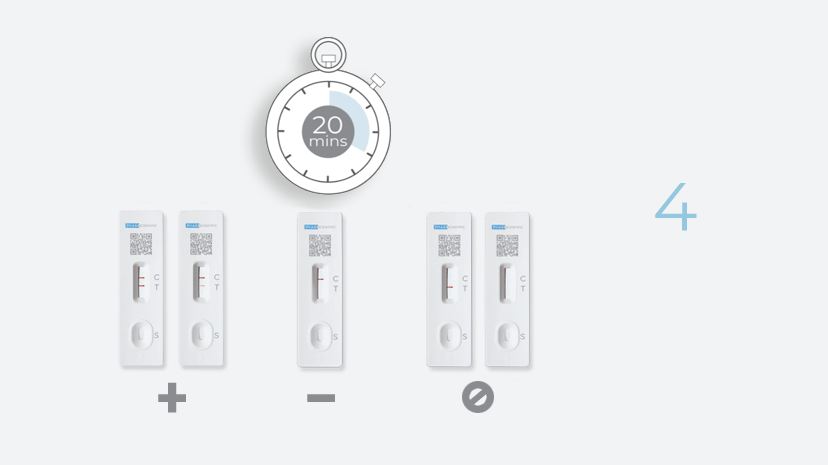
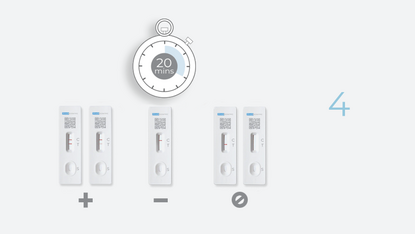
Test Result Interpretation
| CT Indicator | Result | |
|---|---|---|
 | A line appears in regions (C) and (T) | Positive |
 | A line appears in region (C) | Negative |
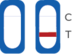 | No line appears in region (C) | Invalid |
Positive
If the control (C) line and the test (T) line are visible, the test is positive. Any faint visible red colored test (T) line with the control (C) line should be read as positive. No serial testing is required if you get a positive result at any point.
Negative
If the control line (C) is visible, but the test (T) line is NOT visible, the test is negative.
To increase the chance the negative result for COVID-19 is accurate, you should:
- Test again in 48 hours if the individual has symptoms on the first day of testing.
- Test 2 more times at least 48 hours apart if the individual does not have symptoms on the first day of testing.
Invalid
No red-colored line next to the "C" means the test is invalid. Re-test with a new swab and a new test device.
Specifications
| Product Code | EUA 210259 |
|---|---|
| Items Per Box | 25 |
| Specimen Type | Direct Anterior Nasal Swab |
| Storage Condition | 36º-86ºF (2º-30ºC) Do not freeze. Avoid direct sunlight. |
| Shelf Life | 15 Months (With 3 Month FDA Extension - Learn More) |
| Accuracy | Sensitivity 88.9%; Specificity 96.8%; Overall accuracy 95.6% |
Principle
The INDICAID COVID-19 Rapid Antigen Test detects antigens from SARS-CoV-2 virus and can be used for COVID-19 screening during active infection.
Intended Use
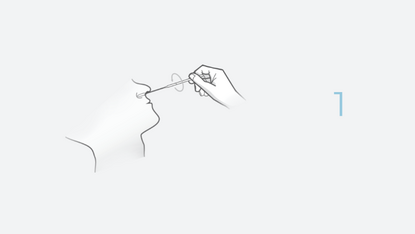
The INDICAID COVID-19 Rapid Antigen Test is a rapid lateral flow immunoassay device intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2. The test is performed by a CLIA Certified Health Care Provider (HCP) using direct anterior nasal swab specimens from suspected COVID-19 individuals within the first five (5) days of symptom onset when tested at least twice over three days with at least 48 hours between tests. For individuals without symptoms or other epidemiological reasons to suspect COVID-19, it is authorized when tested at least three times over five days with at least 48 hours between tests. Anterior nasal swab specimens may be collected by an HCP or self-collected by individuals 18 years of age or older, under the supervision of an HCP.
The INDICAID COVID-19 Rapid Antigen Test is only for in vitro diagnostic use under the Food and Drug Administration’s Emergency Use Authorization. This product has not been FDA cleared or approved.
External Quality Controls (5+/5-)
 The INDICAID COVID-19 Antigen Quality Controls are external liquid quality controls. The controls are specifically formulated and manufactured to ensure that the test’s reagents and materials are working and that the test procedure is correctly performed. The Quality Controls consist of positive and negative control samples that should be run once with every new lot, shipment, and each new user, using the test procedure provided.
The INDICAID COVID-19 Antigen Quality Controls are external liquid quality controls. The controls are specifically formulated and manufactured to ensure that the test’s reagents and materials are working and that the test procedure is correctly performed. The Quality Controls consist of positive and negative control samples that should be run once with every new lot, shipment, and each new user, using the test procedure provided.
It is the responsibility of each laboratory or healthcare setting using the INDICAID COVID-19 Rapid Antigen Test to establish an adequate quality assurance program to ensure the performance of the test kit under its specific locations and conditions of use. Quality control requirements should be followed in conformance with local, state, and federal regulations or accreditation requirements and the user laboratory’s standard quality control procedures.
The Quality Controls Quick Reference Guide
View / Download The INDICAID Quick Reference Guide
Purchase These Important Quality Controls
Purchase 100 BOXES OR LESS (5+/5-)- PURCHASE ONLINE
Purchase the INDICAID QUALITY CONTROLS (<100 PACKS) Online
Purchase OVER 100 Boxes (5+/5-) - PURCHASE VIA SALES PROFESSIONALS
Purchase the INDICAID QUALITY CONTROLS (>100 PACKS) VIA SALES PRO
Frequently Asked Questions
The INDICAID COVID-19 Rapid Antigen Test is a lateral flow immunoassay designed for the qualitative detection of SARS-CoV-2 antigens. Antigen tests are designed to detect proteins from the virus that causes COVID-19 through swab specimens taken from the patient’s nose.
This test can be administered with no equipment or training needed. The test has high sensitivity and can detect lower viral load samples against competitive products. This test has been granted Emergency Use Authorization (EUA) by the United States Food and Drug Administration.
The shelf-life of the INDICAID test is 15 Months from the date of manufacture. The expiration date is provided on the label of the outer box. This includes the 3 Month (90 Day) FDA extension.
Data from our US multi-center clinical study demonstrates similar performance for detection of the COVID-19 Omicron variant compared to the original COVID-19 strain.
The test is performed by a CLIA Certified Health Care Provider (HCP) using direct anterior nasal swab specimens from suspected COVID-19 individuals within the first five (5) days of symptom onset. Anterior nasal swab specimens may be collected by an HCP or self-collected by individuals 18 years of age or older, under the supervision of an HCP.
INDICAID’s intuitive design allows the collection of multiple samples within a very short timeframe, followed by bulk-testing of samples within only two hours.
The swab test method is utilized by the biomedical community for the collection of upper-respiratory specimens for molecular and rapid antigen testing. The CDC supports and promotes this testing method in light of the current COVID-19 pandemic climate for its practicality, simplicity, and efficiency of gathering much-needed data in bulk.
A negative test result means that proteins from the virus that causes COVID-19 were not found in your sample, all negative results are presumptive. However, it is possible for this test to give a negative result that is incorrect (false-negative) in some people with COVID-19. Your healthcare provider will consider the test result together with all other aspects of your medical history (such as symptoms, possible exposures, and geographical location of places you have recently traveled) in deciding how to care for you.
Serial testing should be performed in individuals with negative results at least twice over three days (with 48 hours between tests) for symptomatic individuals and three times over five days (with at least 48 hours between tests) for asymptomatic individuals. You may need to purchase additional tests to perform this serial (repeat) testing.
A positive test result means that proteins from the virus that causes COVID-19 were found in your sample. This means that it is likely that you have COVID-19, even if you do not have any symptoms. There is also a very small chance that this test can give a positive result that is incorrect (a false-positive), particularly when used in a population without many cases of COVID-19. Your healthcare provider will discuss the next steps with you and how to best care for you based on your medical history and symptoms.
• This test does not differentiate between SARS-CoV and SARS-CoV-2 viruses, so you may receive a positive result but have a different coronavirus that does not cause COVID-19.
• It is possible to test a person too early or too late during COVID-19 to make an accurate diagnosis. The amount of antigen in a sample may decrease as the duration of illness increases. Negative results should be treated as presumptive, results from patients with symptom onset beyond 5 days may need to be confirmed with a molecular assay, if necessary, for patient management.
• Improper handling of the test kit not according to guidelines may yield imprecise test results.
• When an incorrect result is suspected, it is important that you engage with your healthcare provider to help you understand the situation and evaluate your next steps.
The INDICAID COVID-19 Rapid Antigen Test is for diagnosis only and is safe to use, with no long-term impact on human health.
Potential risks include:
Possible discomfort or other complications that can happen during sample collection.
Possible incorrect test result (see below for more information.
Potential benefits include:
The results, along with other information, can help your healthcare provider make informed recommendations about your care.The results of this test may help limit the spread of COVID-19 to your family and those you come in contact with.
For detailed information see the “Warnings, Precautions, and Safety Information” section of the Instructions For Use (IFU).
There are different kinds of tests for COVID-19. Molecular tests (also known as PCR tests) detect genetic material from the virus. Antigen tests detect proteins from the virus. Antigen tests are very specific for the virus but are not as sensitive as molecular tests. This means that a positive result is highly accurate, but a negative result does not rule out infection. Due to the lower sensitivity of antigen tests, there is a higher chance this test will give you a false negative result when you have COVID-19 than a molecular test would.
If your antigen test result is negative, serial testing should be performed at least twice over three days (with 48 hours between tests) for symptomatic individuals and three times over five days (with at least 48 hours between tests) for asymptomatic individuals. You may need to purchase additional tests to perform this serial (repeat) testing. All negative results are presumptive.
Due to the lower sensitivity of antigen tests, there is a higher chance this test will give you a false negative result when you have COVID-19 than a molecular test would.
Discuss with your healthcare provider whether a molecular test would help with your care, and when you should discontinue home isolation if necessary. If a molecular test is not available, the CDC recommends that you should stay home.
Refer to the CDC website for most updated guidelines: https://www.cdc.gov/coronavirus/2019-ncov/your-health/isolation.html
Antigen tests are known to be less sensitive than molecular tests that detect viral nucleic acids. Negative results should be treated as presumptive and may be confirmed with a molecular assay, if necessary, for patient management. Results should be considered in the context of a patient’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19.
INDICAID PoC DOWNLOADS
INDICAID Rapid Antigen Test Instruction Guide
Contact Sales & Customer Service
For quantities less than 100, buy online:
For quantities over 100, contact sales:

Customer Service