A Premium At-Home COVID-19 Rapid Antigen Testing Product Hits U.S. Retail Stores
InsightsMarch 28, 2022
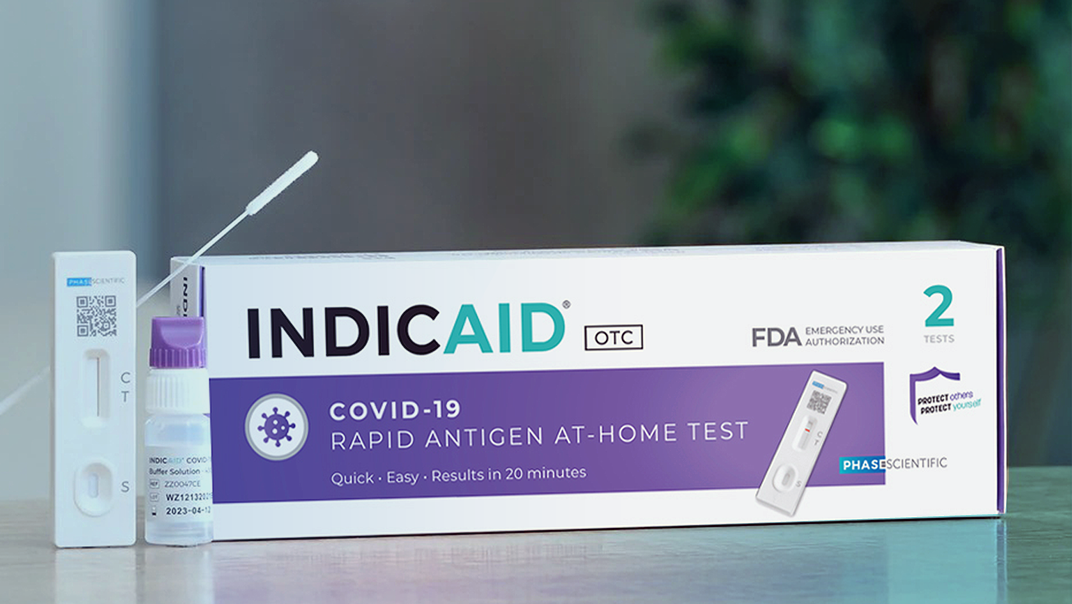
PHASE Scientific’s INDICAID COVID-19 Rapid Antigen Test Obtains FDA Emergency Use Authorization (EUA) for Over-the-Counter (OTC), Asymptomatic, Non-Prescription Use
March 16, 2022 - The biotech conglomerate, PHASE Scientific USA, announced the FDA’s EUA green-light to launch INDICAID COVID-19 Rapid Antigen Over-the-Counter (OTC) tests nationwide. With INDICAID OTC delivered to leading retailers across the country, the American public will now have direct access to the same technology used by doctors and health care professionals without the need for a prescription or a lab.
Individuals over 15 can now perform the test from the comfort of home, the office, or on the go. In as quickly as 20 minutes—and without equipment or training needed - INDICAID Rapid Antigen OTC will provide instant, real-time results. The clear, do-it-yourself box instructions will show consumers how to easily perform a shallow nasal swab. Each box will include two tests indicated for asymptomatic serial testing, where an individual can take the test twice within three days. For symptomatic testing, an individual can take the test once within seven days during the first signs of infection. When administered by an adult, the test can even be used for children as young as two-years-old.
As Chairman and CEO, Ricky Chiu states in his recent interview with the Hong Kong International Business Channel regarding PHASE Scientific’s proprietary technology: “Technologies can improve the diagnostic accuracy and allow a lot of normalized testing to be done … in a lab, a resource-poor setting, or a home test.”
In showing the company’s pledge to meet surges in buyer-demand—especially to further consumers’ peace of mind—PHASE Scientific USA performed a multi-center clinical study providing data demonstrating how INDICAID Rapid Antigen OTC has similar performance to detect the COVID-19 Omicron variant when compared to the original COVID-19 strain. Because it has a shelf-life of one (1) year from the date of manufacture alongside 85% (Sensitivity) PPA: Positive Percent Agreement and 97% (Specificity) NPA: Negative Percent Agreement, the company guarantees to deliver a premium testing product to everyone’s daily life.



