Performance of the INDICAID COVID-19 Rapid Antigen Test Against Omicron Variant (B.1.1.529)
NewsFebruary 1, 2022
The B.1.1.529 variant, termed the Omicron variant, is a highly transmissible SARS-CoV-2 variant of concern. It is important that current SARS-CoV-2 diagnostic tests remain capable of detecting the B.1.1.529 variant. This white paper describes our evaluation of the INDICAID COVID-19 Rapid Antigen Test when used to detect specimens containing the B.1.1.529 variant.
The INDICAID COVID-19 Rapid Antigen Test
The INDICAID COVID-19 Rapid Antigen Test by PHASE Scientific is a US-FDA Emergency Use Authorized (EUA) lateral flow immunoassay designed for qualitative detection of SARS-CoV-2 nucleocapsid protein in direct nasal swab samples. The test produces a simple readout with the presence of a visible test line to indicate detection of the SARS-CoV-2 antigens. In contrast to PCR-based detection methods, INDICAID COVID-19 achieves results in 20 minutes without equipment or sophisticated training required.
INDICAID, PCR, and Sequencing Study Design
The INDICAID COVID-19 Rapid Antigen Test was evaluated in the hands of the lay user at four (4) US-based clinical study sites that were representative of home-use test settings. Samples were collected between November – December 2021 by patients who experienced at least one COVID-19 symptom within 6 days or less from the date of the test. Each patient self-tested with the INDICAID test, while and a second nasal sample was collected by a healthcare professional for subsequent analysis by RT-PCR and sequencing.
The presence of SARS-CoV-2 was determined by RT-PCR with the FDA EUA SARS-CoV-2 Hologic Panther Assay. The samples were then subjected to a variant ID detection workflow that closely resembles the Illumina COVIDSeq NGS Test.
Test Performance
In total, 242 patients were enrolled in the clinical study. The INDICAID COVID-19 Rapid Antigen Test detected 58 patients who were confirmed positive for the B.1.1.529 variant. Cycle threshold (Ct) values for each sample are shown in (Figure 1). The average Ct value was 23.8 (range 17.2 to 33.2).
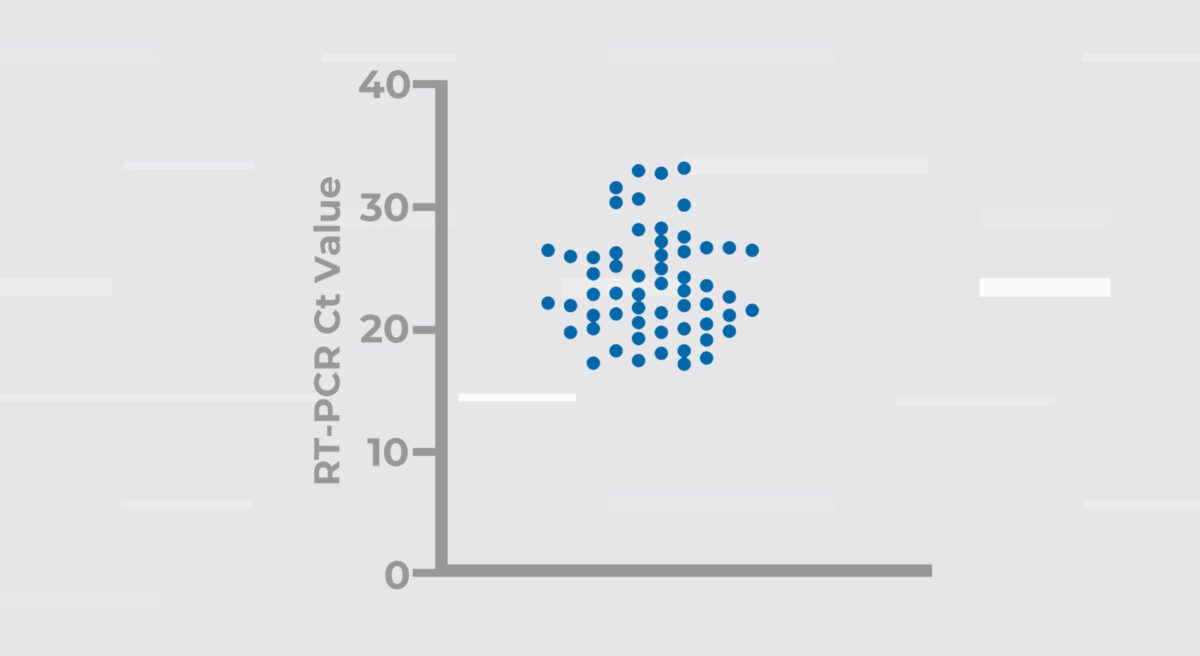
Figure 1. RT-PCR cycle thresholds for 58 SARS-CoV-2 positive samples that were detected by the INDICAID COVID-19 Rapid Antigen Test.
Conclusion
The INDICAID COVID-19 Rapid Antigen Test was able to detect the B.1.1.529 variant, confirmed by whole genome sequencing, in specimens with a wide range of viral loads (RT-PCR Ct range 17.2 to 33.2). Therefore, it can be concluded that the B.1.1.529 (Omicron) variant does not impact the performance of the INDICAID COVID-19 Rapid Antigen test.
Contact us at ussales@phasesci.com



